Organogold(III) complex accumulates in mitochondria of lung cancer cells
Precious metals are not merely ornaments; they are also
important components of pharmaceuticals, like the antitumor drug
cisplatin. Recently, the search for alternatives with improved activity
has begun to focus on gold. In the journal Angewandte Chemie, a
French research team has now published the first study about the
speciation and distribution of an organogold(III) complex in cancer
cells and reveals how specially designed “organogold” complexes might
open exciting avenues for fighting cancer.
© Wiley-VCH, re-use with credit to 'Angewandte Chemie' and a link to the original article.
Gold has a unique electronic structure giving it
exceptional chemical traits that translate into subtle interactions with
biological molecules. Yet, to date, we have little information about how
gold(III) complexes with antitumor activity behave in a biological
environment. Do they change? Are they reduced to gold (I) or metallic
gold? Where in the cell do they attack? Researchers led by Benoît
Bertrand, Michèle Salmain, Sylvain Bohic, and Jean-Louis Hazemann at
Sorbonne Université, the Université Grenoble Alpes, CNRS, INSERM, and
the European Synchrotron Research Facility, have now carried out a
comprehensive study on the chemical reactivity and antitumor activity of
various gold(III) complexes. They used a combination of different
methods based on synchrotron X-ray radiation—very intensive, bundled
flashes of light produced in particle accelerators.
Common to all the complexes they examined (cationic
biphenyl gold(III) complexes with aryl, alkyl, and diphosphine helper
ligands, known as [(C^C)Au(P^P)]+ cations) is a gold atom
bonded to two carbon atoms of the first ligand and two phosphorus atoms
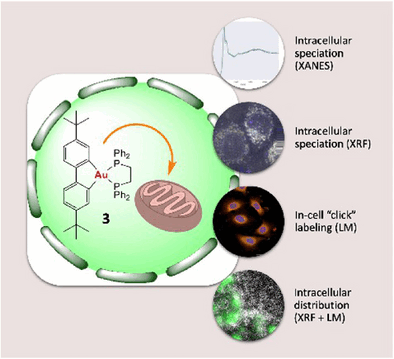
of the second, clasping like two sets of tongs. The analyses demonstrate
that all the complexes examined were stable in both cell-free
environments and inside lung cancer cells. They were not reduced and did
not release their ligands to form new bonds.
The complexes were demonstrated to be toxic against tumor
cells. A “dppe complex” (biphenyl gold(III) complex with
1,2-diphenylphosphinoethane (dppe) ligand) was the most active. The team
used a special setup of synchrotron cryo-X-ray nanoanalysis to “map”
elements including gold in frozen-hydrated lung cancer cells with
nanometer-scale resolution and locate the dppe complex. It was found to
accumulate selectively in the mitochondria, the “powerhouses” of the
cells. The advantage of this method is that no labeling, which could
distort the result, is needed. This gives scientists a unique clarity
when examining cells in their near-native state at the nanoscale.
By using X-ray absorption spectroscopic methods, the team
obtained important information about the valency, geometry, and
oxidation state of the gold atom in the complex. These indicate that the
antitumor activity of the gold complexes primarily stems from the native
cationic species (the [(C^C)Au(P^P)]+ cations). It probably
results from interactions between the whole complex and specific
biological molecules, whose function is disrupted. This differentiates
these drug candidates from other, differently structured gold complexes,
which generally trigger cell death through direct coordination of the
gold center with biomolecules. These results establish a relationship
between the chemical structure and reactivity of a gold complex, its
speciation in the cell, and its cytotoxicity.
(3419 characters)
About the Author
Dr. Benoît Bertrand is a CNRS junior researcher at the Institut Parisien de
Chimie Moléculaire at Sorbonne Université. His research interest is the
development of new organometallic gold complexes and the study of their
anticancer properties with a particular focus on the intracellular
reactivity of the complexes.
Copy free of charge—we would appreciate a transcript/link of your
article. The original articles that our press releases are based on can
be found in our online pressroom.