Electrocatalytic sterilization: nanowires produce localized highly alkaline microenvironments
Harmful microorganisms such as bacteria represent one of the
largest threats to human health. Efficient sterilization methods are
thus a necessity. In the journal Angewandte Chemie, a research
team has now introduced a novel, sustainable, electrocatalytic
sterilization method based on electrodes covered with copper oxide
nanowires. These generate very strong local electric fields thereby
producing highly alkaline microenvironments that efficiently kill
bacteria.
© Wiley-VCH, re-use with credit to 'Angewandte Chemie' and a link to the original article.
Conventional disinfection methods, such as chlorination,
treatment with ozone, hydrogen peroxide oxidation, and irradiation with
ultraviolet light have disadvantages, including harmful by-products and
high energy consumption. Electrochemical disinfection methods, which
rely primarily on a pulsed high-voltage electric field and the
electrocatalytic generation of highly oxidative radicals, are more
efficient and sustainable. However, they require either high voltage or
a significant gas supply, which limits their application in practice.
A team led by Tong Sun and Yuanhong Xu at Qingdao
University (China) have now proposed a novel, in situ,
electrocatalytic sterilization method that induces localized highly
alkaline microenvironments in neutral electrolytes under a constant
current at relatively low voltage. Most bacteria cannot survive in such
extremely alkaline environments.
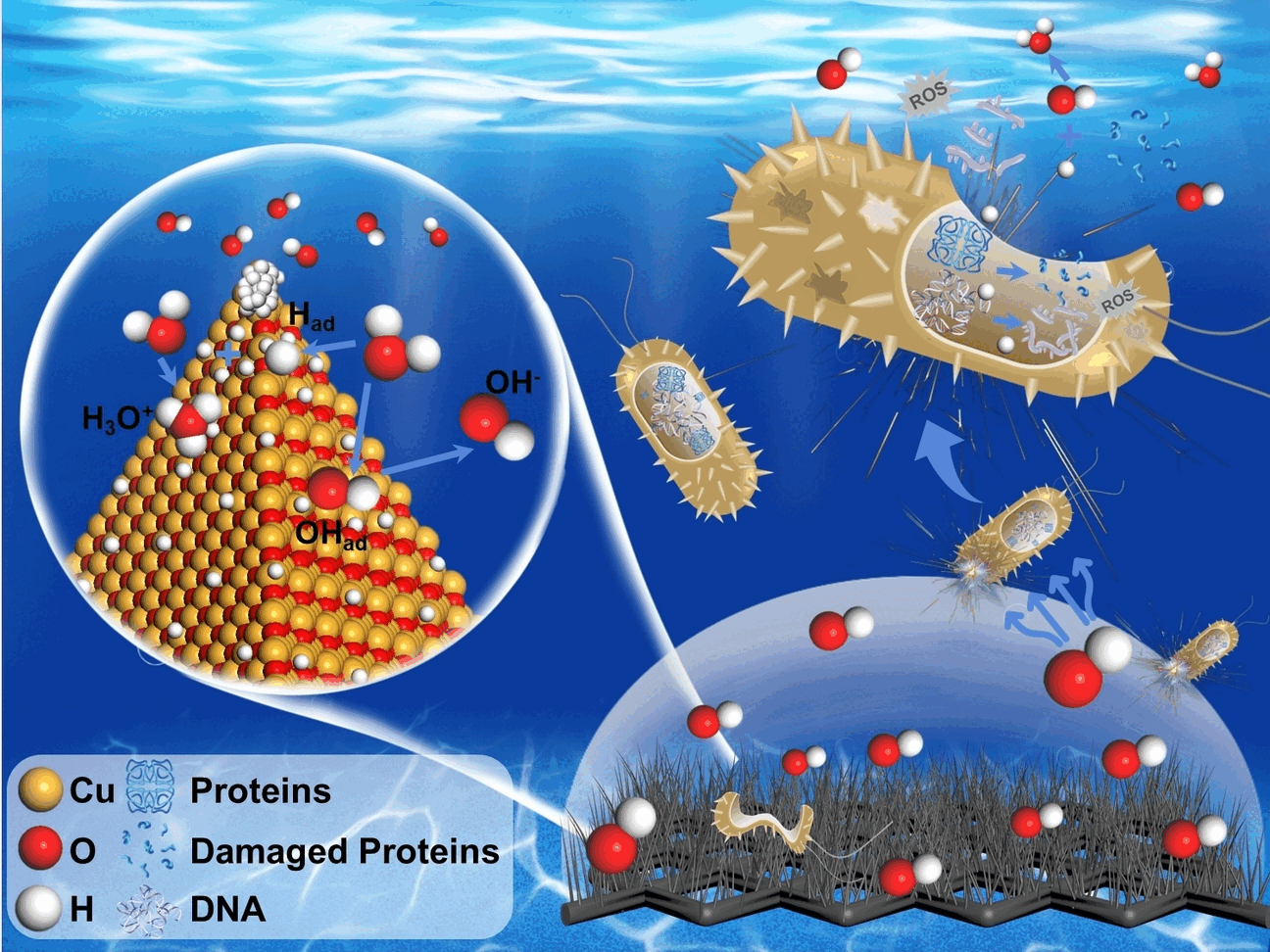
The method is successful owing to cathodes made of a copper
wire mesh that is coated with copper oxide nanowires. On highly curved
structures such as the tips of nanowires, extremely strong local
electric fields can form, allowing electrocatalysts to function very
effectively. At the cathode, the hydrogen evolution reaction (HER)
facilitates the efficient adsorption of hydronium ions (H3O+) by the
nanowires, producing a rapid increase in the hydroxide ion concentration
(OH-) in their immediate surroundings. This produces a
localized, highly alkaline microenvironment. The overall pH value of the
sterilization solution is only slightly increased, so it does not
require neutralization before disposal.
The resulting highly alkaline microenvironment kills off
bacteria within a few minutes, as the team demonstrated with Escherichia
coli (E. coli). The bacteria are killed due to collapse of
protein transport through the bacterial cell membrane because there are
effectively no protons available in this environment. This inhibits ATP
synthesis, resulting in an energy deficit and oxidative stress. In
addition, the NADPH/NAD+ equilibrium, critical for gene
regulation and metabolism, is disrupted. The bacteria die off.
This new approach could be a starting point for the
development of high-performance, nanostructured electrocatalysts for
efficient, environmentally friendly, and safe electrochemical
disinfection strategies for a variety of sterilization applications.
(2965 characters)
About the Author
Dr. Yuanhong Xu is a Professor at the College of Life
Sciences at Qingdao University and has been working in the field of
electrochemistry/biotechnology for more than 20 years. She was honored
with the “Yangtze River Scholar Award” from the Ministry of Education of
the People's Republic of China and is a German Humboldt Scholar. Dr.
Tong Sun is a Professor at the College of Chemistry and Chemical
Engineering at Qingdao University. His research interests are in the
emerging field of nano-electrochemistry, including electrochemical
kinetics, nano- and bio-electrochemistry and scanning electrochemical
microscopy.
Copy free of charge—we would appreciate a transcript/link of your
article. The original articles that our press releases are based on can
be found in our online pressroom.