Ultrasensitive detection of NO using a conductive 2D metal–organic framework
Detection of nitric oxide (NO) is important for monitoring
air quality because the NO released in the combustion of fossil fuels
contributes to acid rain and smog. In medicine, NO is an important
messenger molecule and serves as a biomarker for asthma. In the journal Angewandte
Chemie, a research team now reports a material that can detect NO
reversibly, with low power, and with high sensitivity and selectivity: a
copper-containing, electrically conducting, two-dimensional
metal–organic framework.
© Wiley-VCH, re-use with credit to 'Angewandte Chemie' and a link to the original article.
Metal–organic frameworks (MOFs) are latticelike structures
consisting of metal “nodes” connected by organic bridges (ligands). An
emerging class of MOFs are electrically conducting structures consisting
of layers. These 2D-cMOFs have demonstrated great potential as
chemiresistive sensors that react to the presence of specific molecules
with a change to their electrical resistance, which may allow for
particularly sensitive and low-power detection of toxic gases. Problems
with such systems have included cross-reactivity with a variety of gases
and limited reusability due to irreversible binding of the analytes.
Katherine A. Mirica, Christopher H. Hendon, and their team
at Dartmouth College (Hanover, NH, USA), the University of Oregon
(Eugene, OR/USA), and Ulsan National Institute of Science and Technology
(South Korea), have now developed a reusable 2D-cMOF for the highly
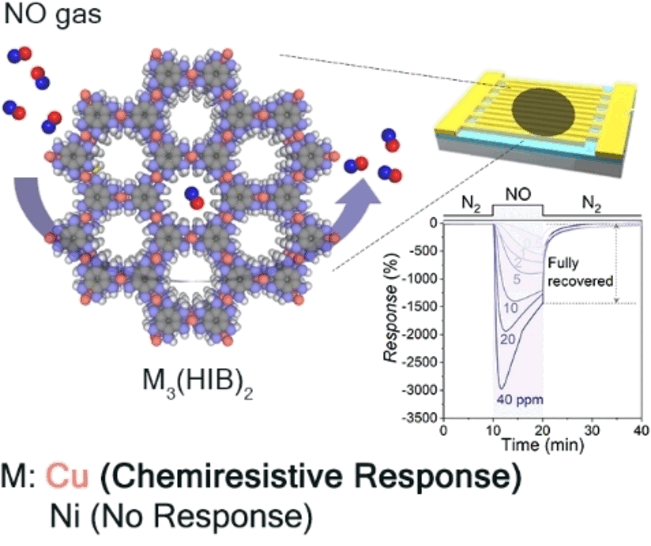
selective detection of NO. They chose to use a 2D-cMOF based on copper
and hexaiminobenzene, Cu3(HIB)2. Thanks to their
different synthetic strategy (the linker was added as an undissolved
powder to a solution of Cu2+ ions and potassium acetate), the
team produced a material with significantly higher crystallinity
(rod-shaped crystallites about 500 nm in length) than has previously
been attained.
The crystallites consist of stacked layers of a weblike
structure of six-membered rings linked together by copper ions bound to
their nitrogen atoms. Spectrometric analyses and computations revealed
that the binding sites for NO were Cu-bis(iminobenzosemiquinone) units
of the copper-2D-cMOFs. An analogous compound made with nickel instead
of copper demonstrated no significant absorption of NO. Evidently,
copper ions with a single positive charge, which are present in small
amounts in the structure besides those with a twofold positive charge,
play an important role in binding NO. Computational studies suggest that
the adsorbed NO significantly distorts the structure, destabilizing the
bound state, which is the primary cause for the desirable reversibility
of the NO adsorption.
This new sensor material detects NO at room temperature and
low voltage (0.1 V) with high sensitivity (detection limit about 1.8
ppb) and could be reused for at least seven cycles without regeneration.
Quantitative measurements of NO were also successful in the presence of
moisture, and showed high enhancement of sensor signal towards NO in
comparison to other gases, such as nitrogen dioxide, hydrogen sulfide, sulfur dioxide,
ammonia, and carbon monoxide and dioxide.
(3153 characters)
About the Author
Dr. Katherine Mirica is an Associate Professor of Chemistry
at Dartmouth College. Her main research direction focuses on the
development of multifunctional porous materials capable for sensing,
filtration, and detoxification of hazardous chemicals.
Copy free of charge—we would appreciate a transcript/link of your
article. The original articles that our press releases are based on can
be found in our online pressroom.