Gamma radiation converts methane into glycine and other complex molecules
Gamma radiation can convert methane into a wide variety of
products at room temperature, including hydrocarbons, oxygen-containing
molecules, and amino acids, reports a research team in the journal Angewandte
Chemie. This type of reaction probably plays an important role in
the formation of complex organic molecules in the universe—and possibly
in the origin of life. They also open up new strategies for the
industrial conversion of methane into high value-added products under
mild conditions.
© Wiley-VCH, re-use with credit to 'Angewandte Chemie' and a link to the original article.
With these research results, the team led by Weixin Huang
at the University of Science and Technology of China (Hefei) has
contributed to our fundamental understanding of the early development of
molecules in the universe. “Gamma rays, high-energy photons commonly
existing in cosmic rays and unstable isotope decay, provide external
energy to drive chemical reactions of simple molecules in the icy
mantles of interstellar dust and ice grains,” states Huang. “This can
result in more complex organic molecules, presumably starting from
methane (CH4), which is widely present throughout the
interstellar medium.”
Although higher pressures and temperatures reign on Earth
and on planets in the so-called habitable zone, most studies of cosmic
processes are only simulated under vacuum and at extremely low
temperatures. In contrast, the Chinese team studied the reactions of
methane at room temperature in the gas and aqueous phases under
irradiation with a cobalt-60 emitter.
The composition of the products varies depending on the
starting materials. Pure methane reacts—with very low yield—to give
ethane, propane and hydrogen. The addition of oxygen increases the
conversion, resulting mainly in CO2 as well as CO, ethylene,
and water. In the presence of water, aqueous methane reacts to give
acetone and tertiary butyl alcohol; in the gas phase, it gives ethane
and propane. When both water and oxygen are added, the reactions are
strongly accelerated. In the aqueous phase, formaldehyde, acetic acid,
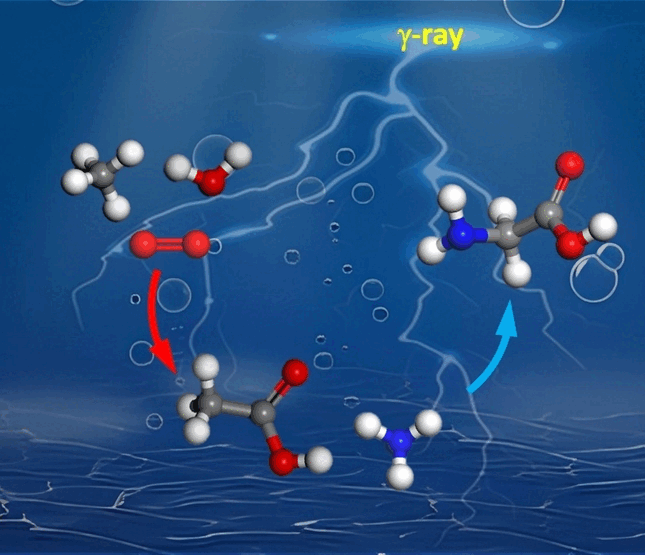
and acetone are formed. If ammonia is also added, acetic acid forms
glycine, an amino acid also found in space. “Under gamma radiation,
glycine can be made from methane, oxygen, water, and ammonia, molecules
that are found in large amounts in space,” says Huang. The team
developed a reaction scheme that explains the routes by which the
individual products are formed. Oxygen (∙O2−)
and ∙OH radicals play an important role in this. The rates of these
radical reaction mechanisms are not temperature-dependent and could thus
also take place in space.
In addition, the team was able to demonstrate that various
solid particles that are components of interstellar dust—silicon
dioxide, iron oxide, magnesium silicate, and graphene oxide—change the
product selectivity in different ways. The varied composition of
interstellar dust may thus have contributed to the observed uneven
distribution of molecules in space.
Silicon dioxide leads to a more selective conversion of
methane to acetic acid. Says Huang, “because gamma radiation is an
easily available, safe, and sustainable source of energy, this could be a
new approach for using methane as a carbon source that can be
efficiently converted into value-added products under mild conditions—a
long-standing challenge for industrial synthetic chemistry.”
(3427 characters)
About the Author
Dr Weixin Huang is the Changjiang Professor in Physical
Chemistry at USTC appointed by the Ministry of Education of China. His
research focuses on surface chemistry and catalysis of solid catalysts
with well-defined structures.
Copy free of charge—we would appreciate a transcript/link of your
article. The original articles that our press releases are based on can
be found in our online pressroom.