Metal-free organic framework for electrocatalytic production of ethylene from carbon dioxide
Use of the greenhouse gas CO2 as a chemical raw
material would not only reduce emissions, but also the consumption of
fossil feedstocks. A novel metal-free organic framework could make it
possible to electrocatalytically produce ethylene, a primary chemical
raw material, from CO2. As a team has reported in the journal Angewandte
Chemie, nitrogen atoms with a particular electron configuration play
a critical role for the catalyst.
© Wiley-VCH, re-use with credit to 'Angewandte Chemie' and a link to the original article.
Ethylene (ethene, C2H4) is an
essential starting material for many products, including polyethylene
and other plastics. Ethylene is produced industrially by the high-energy
cracking and rectification of fossil feedstocks. The electrochemical
conversion of CO2 to ethylene would be a promising route to
reducing CO2 emissions while also saving energy and fossil
resources.
CO2 is very stable, which makes it difficult to
induce into reaction. With the use of electricity and catalysts, it is
currently possible to convert it into C1 chemicals such as
methanol and methane. The additional challenge in producing ethylene is
that a bond must be formed between two carbon atoms. This has previously
only been achieved with copper catalysts. Metal-free electrocatalysis
would be advantageous because metals are a cost factor and can cause
environmental problems.
A team led by Chengtao Gong and Fu-Sheng Ke at Wuhan
University, China, has now developed a metal-free electrocatalyst for
the conversion of CO2 to ethylene. The catalyst is based on a
nitrogen-containing covalent organic framework (COF). COFs are a new
class of porous, crystalline, purely organic materials with defined
topology. In contrast to metal-organic frameworks (MOFs), they require
no metal ions to hold them together. Their pore sizes and chemical
properties can be tuned over a wide range through selection of the
building blocks.
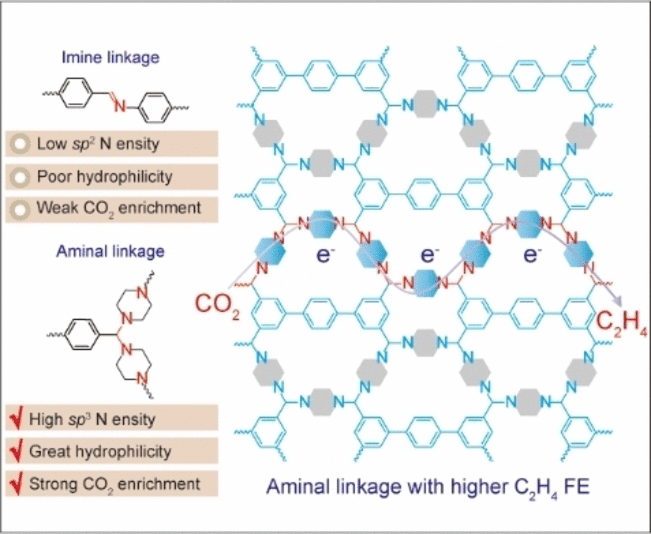
The new COF contains nitrogen atoms with a special electron
configuration (sp3 hybridization) as catalytically active
centers. These sp3 nitrogen centers bind the individual
building blocks into a framework through an aminal link (two amino
groups bound to one carbon atom). In contrast to COFs with a classic
imine-linkage (–C=N–), aminal COFs have strict requirements regarding
the lengths and angles of the bonds between building blocks, which
causes the frameworks to be formed through ring closures. The
researchers found a suitable combination by using piperazine (a
six-membered ring made of four carbon and two nitrogen atoms) and a
building block made of three aromatic, six-membered carbon rings. When
used as electrodes, their new COFs demonstrated high selectivity and
performance (Faraday efficiency up to 19.1%) for the production of
ethylene. Success of the aminal COFs is due to the high density of
active sp3-nitrogen centers, which both very effectively
capture CO2and transfer electrons. This results
in a high concentration of excited intermediates that can undergo C–C
coupling. In contrast, a variety of imine-linked COFs, which contain sp2
nitrogen instead of sp3, were similarly tested and produced
no ethylene. This proves the importance of proper electron configuration
for the electrochemical reduction of CO2 to ethylene.
(3294 characters)
About the Author
Prof. Fu-Sheng Ke is an associate professor at the School
of Chemistry and Molecular Sciences, Wuhan University, focusing on
electrochemical energy storage and conversion.
Copy free of charge—we would appreciate a transcript/link of your
article. The original articles that our press releases are based on can
be found in our online pressroom.