Flexible Sodium-Ion Batteries Improved by Electrolyte Methylation
Flexible aqueous batteries, such as those used in portable
electronics, often contain a hydrogel electrolyte containing water and
salt. Using a chemical modification inspired by nature, a team of
Chinese researchers have now significantly increased the salt stability
of hydrogels used in sodium-ion batteries. A simple methylation of the
hydrogel’s structural polymer prevented salting-out and improved battery
capacity and cycling performance, the team report in the journal Angewandte Chemie.
© Wiley-VCH, re-use with credit to 'Angewandte Chemie' and a link to the original article.
Sodium-ion batteries are a promising alternative to lithium-ion
batteries, since they contain cheaper and more eco-friendly materials
than Li-ion batteries. However, new batteries require the development of
many new components, all of which have to be adapted to the sodium ion.
One of the most essential components is the electrolyte, which in the
case of thin, flexible batteries, is often in the form of a hydrogel.
These flexible, water-containing materials absorb dissolved sodium salts
and can conduct ions.
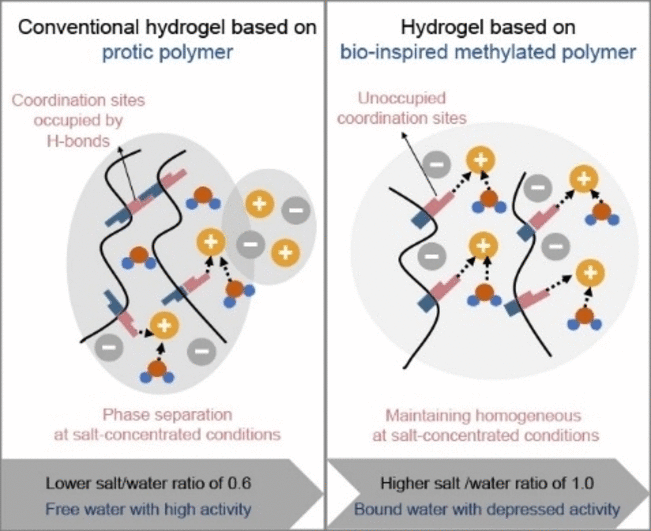
Despite the suitability of hydrogels, an as-yet unsolved problem is
phase separation and salting out at the high salt concentrations needed
for a broad electrochemical stability window. Guanglei Cui and
colleagues from the Chinese Academy of Sciences in Qingdao, China have
now succeeded in modifying a hydrogel for a sodium-ion battery to make
it absorb considerably more salt in a stable and secure manner.
To achieve this, they turned to a technique also employed in nature
for the regulation of water- and salt-binding in large biomolecules:
methylation. In proteins, methylation causes the “capping” of amine and
amide groups, which become less accessible for water molecules that play
a role in cross-linking within the protein structure and the
dissolution of salt ions.
As the polyamide polymers used for hydrogels also contain amide
groups, their extensive cross-linking through water molecules can cause
salting out, which leads to the breakdown of the electrolyte. With this
in mind, the team compared a hydrogel made of a common polyamide to a
hydrogel made of a polyamide with methylated amide groups. The latter
was able to absorb significantly more salt than the original variant.
Even at record-high salt concentrations, the hydrogel electrolyte
remained transparent and stable.
The higher salt content means that the electrochemically usable
voltage range of the cell can be expanded. In addition, the team did not
observe any signs of disintegration at the electrodes, better cycling
stability and the assembled battery cell achieved a greater capacity
than the non-methylated variant. It was even possible to use inexpensive
aluminum foil as a current collector in this system.
The authors suggest that simple polyamide methylation could also be
suitable for other technologies, for example, in drug development, to
make hydrogels more resistant to salts and therefore more stable.
(2996 characters)
About the Author
Guanglei Cui is a
professor at the Qingdao Institute of Bioenergy and Bioprocess
Technology at the Chinese Academy of Sciences, Qingdao, China. His
research interests mainly focus on low-cost energy storage systems,
solid-state batteries, deep-sea power supply systems, and photoelectric
conversion devices.