Some reactions in the reverse Krebs cycle can also be run under meteorite catalysis
Naturally occurring chemical reactions may have evolved into the
biochemical processes we know today. A team of researchers has now
discovered that a reaction sequence from the so-called reverse Krebs
cycle—a fundamental biochemical process—can also take place without
enzymes. The team writes in the journal Angewandte Chemie that metals and even powdered meteorite material can catalyze the hydrogenation reactions.
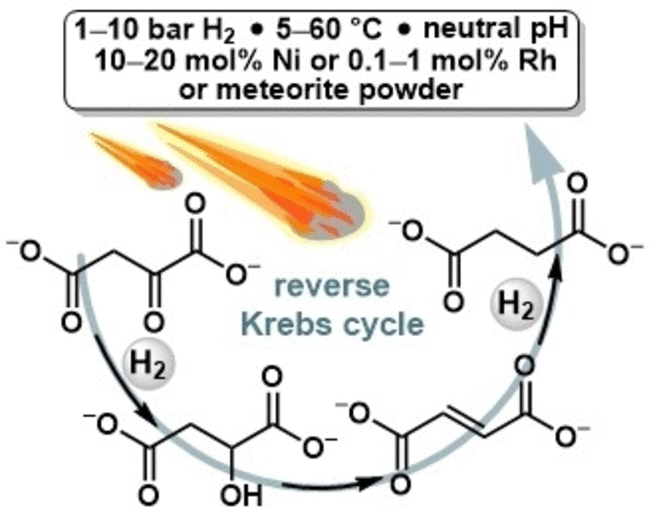
© Wiley-VCH, re-use with credit to 'Angewandte Chemie' and a link to the original article.
Cells obtain energy and molecular building blocks through metabolic
processes. Compounds are synthesized and broken down again in universal
biochemical processes with the help of enzymes. However, simple organic
molecules such as organic acids, amino acids, and peptides have been
found in extraterrestrial objects, suggesting that organic molecules
might have existed on early Earth, even before life as we know it
developed.
Taking the theory of a self-organizing chemical network, some
fundamental biochemical reaction sequences derived from naturally
occurring chemical reactions may have evolved into the biochemical
processes we know today. Sophia Rauscher and Joseph Moran of the
University of Strasbourg, France, have now investigated a sequence of
the reverse Krebs cycle, a biochemical process used by some
microorganisms for fixing carbon dioxide. In this portion of the
process, the small organic molecule oxaloacetate is hydrogenated and
dehydrated to give succinate in three chemical steps.
In the cellular reverse Krebs cycle, hydrogenation—the attachment of
hydrogen atoms—takes place using enzymes that transfer organically bound
hydrogen. In order to simulate hydrogenation as it might have occurred
on the primordial earth three to four billion years ago, Rauscher and
Moran used elemental hydrogen and metal catalysts. They justified these
choices since hydrogen is formed in natural geological processes and can
accumulate in reservoirs in the ground or in hydrothermal vents. In
addition, meteorites that fell to earth during this period brought
metals with them.
In the experiment, malate was initially formed from oxaloacetate in a
hydrogenation reaction, even under mild reaction conditions, mimicking
the first hydrogenation step in the pathway. Following the dehydration
of malate to fumarate, succinate was formed from fumarate in a further
hydrogenation step, following the same sequence of molecules and
reactions as in the biological reverse Krebs cycle. Metals such as
nickel and even pure powdered meteorite sample were able to catalyze the
reactions. These findings could prove relevant for our understanding of
the origins of some fundamental metabolic pathways, the authors argue.
(2713 characters)
About the Author
Dr. Joseph Moran is a
professor of chemistry and catalysis at the University of Strasbourg
& CNRS. The Moran Research Group explores self-organized reaction
networks to understand the origin of life; in particular, the evolution
of biochemical pathways.
Copy free of charge—we would appreciate a
transcript of your article. The original articles that our press
releases are based on can be found in our online pressroom.